Clinical Trials
In a Proof-of concept study, QRX003 was evaluated as adjuvant therapy with systemic biologics. Below is data and photographic evidence from the first patient enrolled in the study dose twice a day with 4% QRX003 Lotion for twelve-week period. Final visit was at 16 weeks, 4 weeks after discontinuation of treatment.
Table 1: First Patient Data from CL-QRX003-002 Open-Label Study Part B- Dosed Twice Daily with QRX003
| End Point | Baseline | 6 weeks | 12 weeks | 16 weeks |
|---|---|---|---|---|
| M-IASI* | 18 | 4 | 3 | 18 |
| WINRS** | 7 | 4 | 2 | 8 |
| IGA*** | Moderate | Mild | Almost Clear | Moderate |
|
*M-IASI: Modified Ichthyosis Area of Severity Index, a score used to assess the severity and extent of skin symptoms associated with ichthyosis. Lower scores indicate improvement.
**WINRS: Worst Itch Numeric Rating Scale, which measures the severity of itch on an 11-point scale (0 = no itch, 10 = worst imaginable itch).
***IGA: Investigator’s Global Assessment, which uses descriptive categories (e.g., clear, mild, moderate, severe) to evaluate the overall severity of Netherton Syndrome symptoms.
|
||||
Photographs illustrating the change in the subject’s skin appearance from Figure 1 at baseline compared to Figure 2 at visit four after 12 weeks of twice daily dosing with QRX003
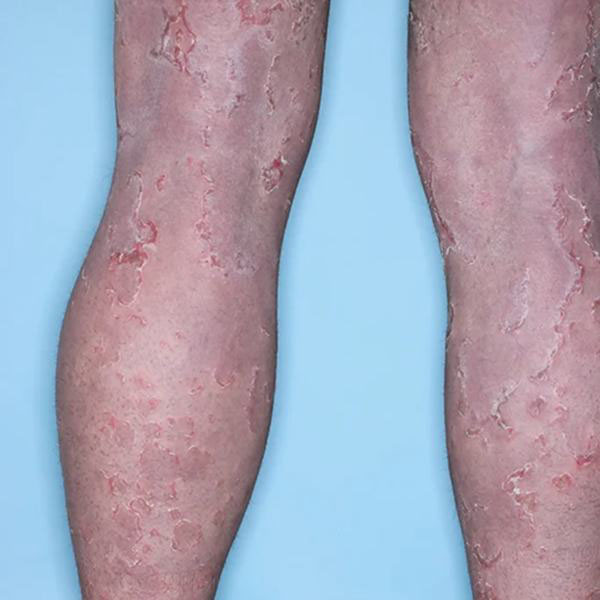
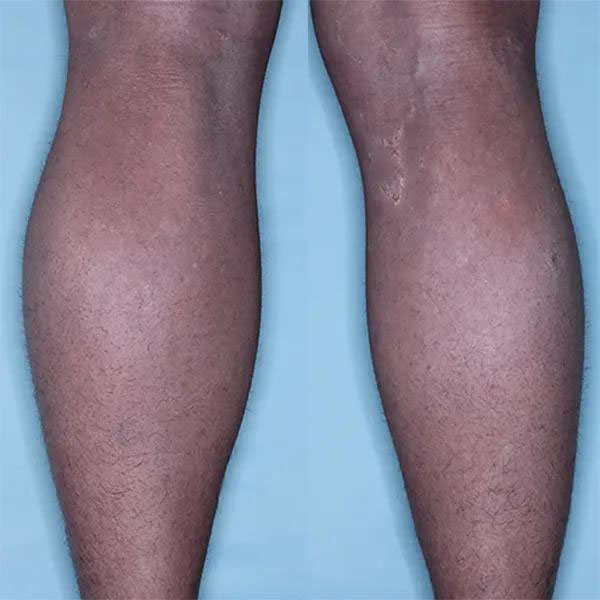
Importantly, upon discontinuation of treatment, all symptoms reverted to baseline. This data supports that the mechanism of action of QRX003 is a competitive broad spectrum serine protease inhibitor and emphasizes that on-going chronic treatment is essential for continued positive clinical outcome.
Current Trials:
Quoin Pharmaceuticals is currently conducting two pivotal trials for Netherton Syndrome.
For more information about Quoin’s clinical trials, please visit https://www.nethertonsyndromeclinicaltrials.com
For more information about current clinical trials, please visit www.clinicaltrials.gov.

