Therapeutic Development Pipeline
| Product Candidate | Indication | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | Approval |
|---|---|---|---|---|---|---|
| QRX003 |
|
|||||
|
||||||
|
||||||
|
||||||
| QRX008 |
|
|||||
| QRX009 |
|
** Clinical trial initiated
*** Clinical testing to commence 1H2026
Product Candidate: QRX003
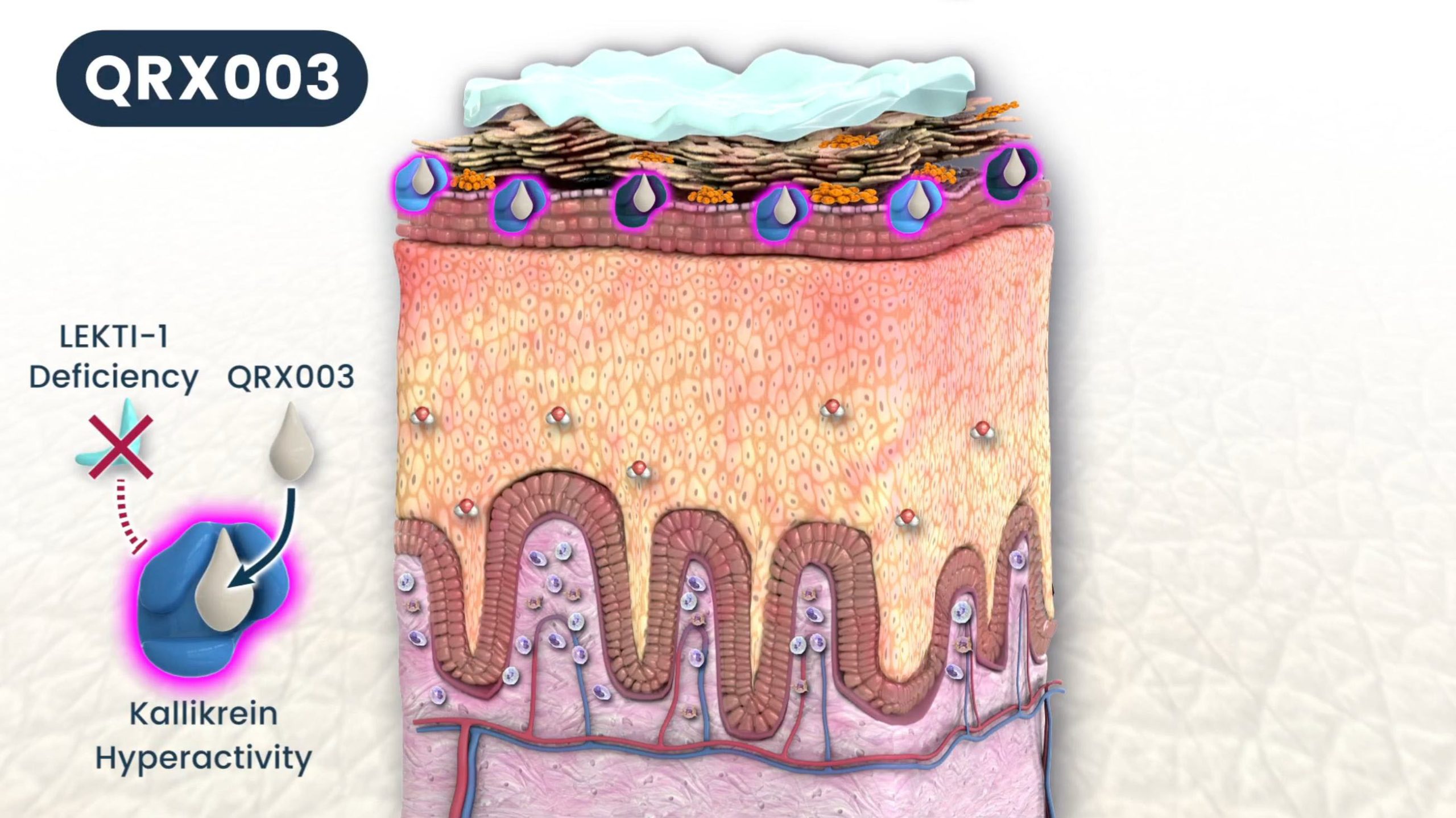
QRX003 is under development for a number of indications. The product is a topical lotion comprised of a broad-spectrum serine protease inhibitor, formulated with the proprietary delivery technology. Our lead indication is Netherton Syndrome (NS).
Target Indications
Netherton Syndrome
Netherton Syndrome – a rare, hereditary skin disorder – is an autosomal recessive genetic disease caused by a mutation in the SPINK5 gene (serine protease inhibitor, Kazal Type 5).
The SPINK5 gene, when behaving normally, encodes a protein called Lympho-epithelial Kazal-type-related Inhibitor, or LEKTI, that serves to regulate the activity of certain skin proteases, called kallikreins, that are responsible for the normal everyday process of skin shedding. For patients with NS, the LEKTI protein is absent, resulting in unregulated hyperactivity of the kallikreins, which in turn results in uncontrolled desquamation and leads to a highly porous and defective skin barrier.
People with Netherton Syndrome have too few layers of their outer skin (stratum corneum), so the skin does not perform its primary function as a protective barrier. This leads to the increased risk of infections, warts, skin cancer, and irritation by allergens and other environmental microorganisms. The skin is prone to scaling and is accompanied by hair anomalies, along with increased susceptibility to atopic eczema. Pruritus (itching) is a major problem as well as elevated IgE antibody levels. Furthermore, because the skin is so compromised, patients experience trans-epidermal water loss (TEWL), and adults can lose up to 2 quarts of water a day.
Currently, there is no cure for Netherton Syndrome, nor are there any approved therapeutic treatments.
Peeling Skin Syndrome
Due to mutations in the TGM5 gene, inherited in an autosomal recessive pattern, this disorder is characterized by the continuous peeling of the top layer of skin. It can appear as abnormal blistering of the skin anywhere on the body, but most often presents on the hands and feet. Symptoms can include pruritus (itching), hyperpigmentation, and redness. Incidence is less than one in one million.
SAM Syndrome
Presents with severe skin dermatitis, multiple allergies and metabolic wasting (SAM). This is a rare life-threatening inherited condition caused by mutations in the desmoglein 1 gene.
Ichthyosis
Ichthyosis refers to a group of more than 30 skin diseases. Typically characterized by dry, scaling, red, itchy skin, the symptoms will vary depending on the type of ichthyosis.
Additional Quoin Programs
VLA-4 inhibition for treatment of Scleroderma
Systemic sclerosis (scleroderma) is a multisystem, autoimmune disorder defined by progressive vascular, inflammatory, and fibrotic dysfunction. It is characterized by an early inflammatory phase, which is followed by progressive fibrosis of varying extent and distribution, resulting in severe dysfunction of involved organs. Interaction of vascular cell adhesion molecule 1 (VCAM-1) with its ligand, very late antigen 4 (VLA-4), is a key process in inflammatory disease. There is an established genetic and clinical link for VCAM-1 in scleroderma, with VCAM-1 having a genome-wide association with scleroderma, and scleroderma patients having elevated VCAM-1 serum levels. VLA-4, an α1β4 integrin, plays a pivotal role in controlling immune cell migration into inflamed tissue as it directly participates in cell arrest under flow. The VCAM-1:VLA-4 interaction therefore makes an attractive target for therapeutic intervention in scleroderma. A lead series of small molecule inhibitors has been developed to disrupt the VCAM-1:VLA-4 interaction. Proof of concept has been demonstrated with VLA-4 inhibition in the Tsk1/+ mouse model of scleroderma. Further preclinical assessment is underway for the selection of a lead compound for entry into the clinic.
Topical Rapamycin Formulations for Rare Vascular and Skin Malformations
QRX009 is a proprietary topical formulation of rapamycin (sirolimus) in development as a potential treatment for a group of rare, disfiguring conditions, including microcystic lymphatic malformations (MLM), venous malformations (VM), and cutaneous angiofibromas, among others. These diseases can lead to significant cosmetic and functional impairment, and there are currently either none or limited FDA-approved topical therapies for these indications.
QRX009 is designed to optimize local skin penetration and deliver rapamycin directly to the affected dermal layers where it can be most effective. Previous clinical observations strongly suggest that limited drug delivery has contributed to the underperformance of other topical rapamycin products. QRX009 is intended to address this challenge by optimizing the retention and absorption of the rapamycin directly at the target site. Quoin is currently evaluating several technologies to determine the optimal delivery system.
Quoin plans to submit Investigational New Drug (IND) applications for at least two of these indications with clinical development expected to commence in 1H 2026.
Venous Malformations (QRX009)
Cutaneous venous malformations (CVMs) are vascular lesions that develop before birth due to a genetic error in how blood vessels form. They are composed of abnormally dilated veins with weak walls and very little muscle support. CVMs typically present as a distinct birthmark on the skin, but their size, appearance, and location can differ significantly from person to person. Currently, there are no FDA approved medications specifically for treating these malformations.

Angiofibroma (QRX009)
An angiofibroma is a benign tumor composed of dilated blood vessels and fibrous connective tissue that can appear as small, firm, red or pink, dome shaped bumps on the skin, or as larger, complex tumors like the juvenile nasopharyngeal angiofibroma (JNA). While often harmless, some may grow large, cause repeated bleeding, or be associated with genetic syndromes like tuberous sclerosis, requiring medical treatment such as surgery or hormone therapy
Quoin Pharmaceuticals is currently managing three concurrent clinical studies to evaluate the therapeutic potential of QRX003 for individuals with Netherton Syndrome.
Patients & Families
Rare diseases are only rare if you don’t live with one.
Quoin has a singular mission of developing safe and effective treatments for patients who live with a rare disease. Put simply, our goal is to provide hope where there is currently none. Patients are the center of everything we do, and we will work tirelessly to deliver on our promise of making our products available to ‘every patient, everywhere.





Resources
FIRST Skin Foundation for Ichthyosis and related skin types
Supports patients and families affected by Ichthyosis.
Phone: (215) 997-9400
Toll free: (800) 545-3286
Website: https://www.firstskinfoundation.org/
NORD National Organization for Rare Disorders
NORD is leading the fight to improve the lives of patients with rare diseases.
Phone: (202) 588-5700
Website: https://rarediseases.org
Genetic and Rare Diseases (GARD) Information Center
GARD is a program of the National Institutes of Health (NIH) that provides free access to reliable, easy to understand information about genetic and rare diseases.
Phone: (301) 251-4925
Toll free: (888) 205-2311
Website: https://rarediseases.info.nih.gov/GARD
Molecular Diagnostic Testing for Netherton Syndrome
Gabriele Richard, MD Associate Professor, Department of Dermatology and Cutaneous Biology and Department of Medicine / Division of Medical Genetics Thomas Jefferson University
Phone: (215) 503-825
Email: Gabriele.Richard@jefferson.edu
Ichthyosis Support Group
Committed to the ongoing provision of an information network and support structure for individuals and families affected by ichthyosis.
Phone: +44 (0) 800 368 9621
Email: isg@ichthyosis.org.uk
Website: https://www.ichthyosis.org.uk/

